News

FIMEA Authorizes GlucoModicum to Conduct Pre-Pivotal Clinical Investigation Under EU MDR Article 62

Eurofins Electric & Electronics Finland audits GlucoModicum-Sofio operations, with zero deviations.
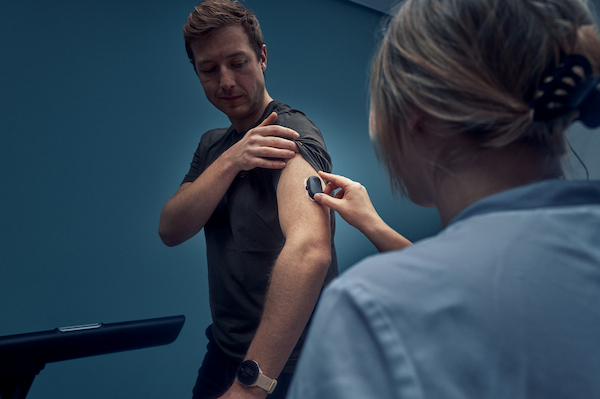
GlucoModicum Completes Clinical Performance Study of 646 Subject Visits and Reports Positive Results for Its Sofio Needle-Free Glucose Monitor

Major Medical Device Development Program Conducted with Phillips-Medisize Across the US and Europe to Advance Sofio

GlucoModicum Appoints Peter Gerhardsson to Board of Directors

GlucoModicum Receives European Commission Seal of Excellence and Funding from the European Innovation Council (EIC)

FIMEA Authorizes GlucoModicum to Conduct Pre-Pivotal Clinical Investigation Under EU MDR Article 62

Eurofins Electric & Electronics Finland audits GlucoModicum-Sofio operations, with zero deviations.

GlucoModicum Completes Clinical Performance Study of 646 Subject Visits and Reports Positive Results for Its Sofio Needle-Free Glucose Monitor

Major Medical Device Development Program Conducted with Phillips-Medisize Across the US and Europe to Advance Sofio

GlucoModicum Appoints Peter Gerhardsson to Board of Directors

GlucoModicum Receives European Commission Seal of Excellence and Funding from the European Innovation Council (EIC)